Search the Community
Showing results for 'Radium'.
-
Identifying watch for possible radioactive concern
Geotex replied to kr2's topic in Identify This Movement or Watch
But the radium wasn’t pure when put on the watch, so it’s already a mixture. We certainly can see counts from watches that penetrate materials that would stop alpha radiation. Again, I’m not paranoid about it, but it shouldn’t be completely discounted when people ask about it. -

Identifying watch for possible radioactive concern
Waggy replied to kr2's topic in Identify This Movement or Watch
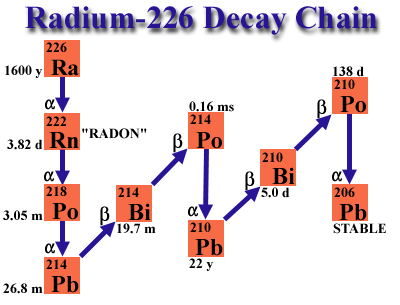
Here is the radium decay chain, as you can see it decays into alpha emitters until you get to lead, but it's first decay product is radon with a half life of 1600 years, so we will all be long dead before the radium on our watch starts emitting anything we need to be overly concerned about. -
Identifying watch for possible radioactive concern
Geotex replied to kr2's topic in Identify This Movement or Watch
Isn't it most correct to say that the mixture of radium decay pathway compounds found in old lume will produce both alpha and beta particle radiation as well as gamma rays, so it's over-simplistic to dismiss it all as low-energy particle emission? True, a cheap geiger counter chirping away with a raw CPM count won't specifically give you the relevant biological dose, but it makes it easy to tell the difference between your local background, a watch with a bit of activity, and one that is comparatively quite active. Each person can make their own risk analysis based on their understanding and concern, but there's nothing wrong with using an inexpensive meter to help make the determination. -

Identifying watch for possible radioactive concern
ScrewDropper replied to kr2's topic in Identify This Movement or Watch
To be honest,I would not rely on cpm for a measurement, particularly with radium. The radium is a particle radiation and this cannot penetrate the skin. It has to be ingested (i.e. inhaled) to have an affect. A more reliable measurement of this kind of radiation is the Sievert, which, roughly, is the affect of radiation. Broadly speaking, when dealing with radium, precautions such as breathing equipment and removing radium in a fluid is advised. Disposal is also something to consider. It's generally a pain in the arse to dispose of and you are better just leaving it undisturbed -

Should I be worried about radium?
LittleWatchShop replied to danizzz's topic in Chat About Watches & The Industry Here
I have said it before, my anecdotal evidence dispels any fear of radium in the quantities we deal with in the watch world. -
I'm a little concerned reselling a watch I think was radium that has dissipated the luminous material. I guess the radium is still active if they took it apart somehow or broke the crystal. I don't see any warnings associated with vintage Seikos etc. I'm still mulling what to do with Dad's radium elgin and my old U.S. Navy radium watch.
-
I've run across a "STAUER" tritium watch that seem pretty bright. I'm looking at a Marathon as well. I had hoped a Radium dial watch would still work but I think all the old Seikos with radium have burned up the luminous material by now. The Ball watches, maybe $1000 , um wow certainly better than Rolex but 3 times the price of a Marathon, couldn't be worth it. (for my worst case scenario we're hunting, rainy, no chance to charge a lume watch, someone is injured at night. I want a watch I can check pulse, hold pressure on a wound for 10 minutes , mark a tourniquet etc.) (and it still works after I've shot my 12 ga. and fell out of a tree). I am having so much fun investigating, collecting and repairing watches !! Not ready for prime time but watches are cool.
-
Those case #'s are from the 3rd and final iteration of the Benrus waterproof design. They had some other case designs in their final years as the quartz crisis came on, but by that time US legal restrictions made them change to advertising only as water "resistant.": There is no gasket, glue, sealant, crystal ring or separate bezel, just a tight fitting flexible crystal meant to fit the machined groove on the case and be installed (and removed) from the front with a crystal lift or wrench. There is a gasket in the crown. Their waterproof guarantee always included the disclaimer that the case, crystal and crown needed to be intact for this functionality. Not sure how these did on a pressure tester, but it was good enough to secure multiple government contracts for a series of military watch models. Most of the civilian cases cover the joint in the split stem, so you can't manipulate them to slide in and out from the side (as you can with some of the military models), you just have to trust the system and yank on the crown! I enjoy working on that era of Benrus watches. Their ETA movements are reasonable to service, they have parts that can still be sourced, and most were tritium lumed so the dials have nice patina without radium burn marks.
-
Should I be worried about radium?
Geotex replied to danizzz's topic in Chat About Watches & The Industry Here
NOS radium lume that has been protected from moisture/humidity is much greener than I would have expected, based on some well-wrapped example hand cards I’ve come across. Very similar to 1st generation “cold” lume and other glow-in-the-dark items from the 70’s and 80’s. -
Should I be worried about radium?
JohnR725 replied to danizzz's topic in Chat About Watches & The Industry Here
One of the things that I find interesting with a vintage new old stock hands still mounted in the paper is the effect of the radium on the paper. In the image I snipped out you urge you can see it but maybe it's the lighting because usually the paper looks much more brown if it's been under radium hand it has a nice light burnt look. -

Should I be worried about radium?
mikepilk replied to danizzz's topic in Chat About Watches & The Industry Here
I'm probably over cautious when removing radium. I cover the work area with cling film and wear latex gloves and an N95 mask. All the disposables I use (cling film, gloves, buds, tissue, water, which I soak up with kitchen roll) get bagged in ziplock bag and binned. I bought a "Pocket Geiger" a few years ago (they were developed for Fukushima) which plugs in to mobile and has an app. It only detects gamma, but It seems quite sensitive. ebay -

Should I be worried about radium?
Jon replied to danizzz's topic in Chat About Watches & The Industry Here
My student brought in his Geiger counter the other day and I put the tray of radium hands under it and the clicking got to a continuous crackle and some pretty high readings. That level of radiation is only problematic at close proximity. It's more the radon and breathing in dust when working with them that is the problem. Radium exposure.pdf Yeah, interesting effect of the radiation on the card. You get it on vintage watches that haven't been used in many years with a faint mark where the hand was Where do you get that from? -

Should I be worried about radium?
Jon replied to danizzz's topic in Chat About Watches & The Industry Here
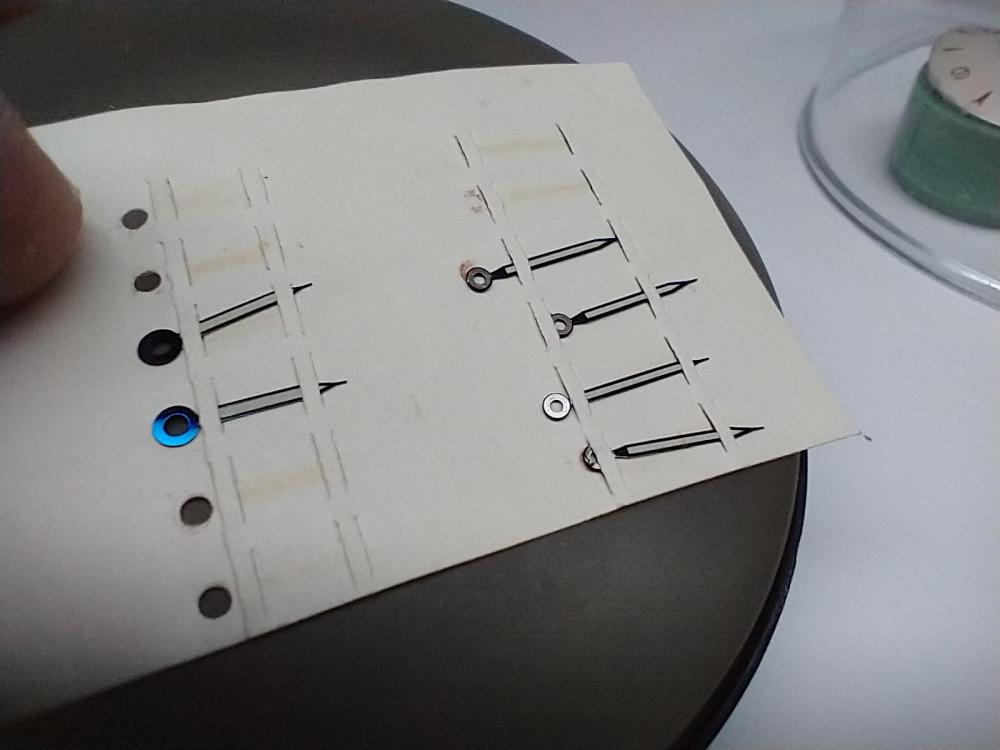
I've got a bit of a collection of NOS vintage radium hands in pristine condition and they have a very mat dull lime green/yellow colour to them. I've tried taking photos of them, but the colour doesn't really show properly in them! They look more white in the photos, which they're not. I tinted the second photo to get the colour of the lume right, although it all depends on your screen. The hand in the middle and at the top is the most like the colour -

Should I be worried about radium?
mikepilk replied to danizzz's topic in Chat About Watches & The Industry Here
While we are on the subject - It reminded of the my one radium watch, my Universal Geneve cal 267. I've always wondered about how (and where) to store it. I had it sealed in plastic bag to contain the radon. But then I read about how that leads to contamination of all the contents by daughter decay elements. This morning I decided to remove the radium to make the watch more safely storable and wearable. As usual, it came off easily with a wet bud. I happen to have an old scrap spare dial from a cal 267, and found (under microscope using a fine oiler) I could easily apply luminova to the numbers to look at least as good as the original. So my question is - What colour should I use. What was the original colour of radium paint? -
I'm looking for a high quality waterproof watch that can be visible after days in the dark, luckily just before I clicked 'buy' on this economical Submariner by Rolex I saw it was only 'luminous'. You would think for that kind of money they could afford Radium or Tritium. ! POS I guess. The best I've seen is the Luminox Navy Seals with tritium tubes. (While the only decent 'luminous' was the Invicta automatic that did hold light for over 12 hrs). I'm still researching. Almost sold my house to get the Rolex, but it's just not good enough. Any suggestions?
-
.thumb.jpg.cb17a66989f1e796fd4217db2e9ca9df.jpg)
Should I be worried about radium?
Knebo replied to danizzz's topic in Chat About Watches & The Industry Here
Wow @RichardHarris123, I didn't know you were a famous scientist! You are one of the authors, right? I watched this a few years ago. What I found interesting is the title of her presentation "how I stopped worrying about radium dials"...... BUT then she carries her watches in a lead-line bag!?!? Sounds like a contradiction to me. Personally (!!!!), I don't want to work on radium dials/hands. Which is a shame, because I love watches from the 1930s to 50s. Even when working on tritium dials, I wear a mask, wear cots (as always..), open the window, try to remove any loose lume with Rodico and then dispose of it, and wash my hands thoroughly. I assume that this level of precaution may be excessive (for Tritium), but I would think that it's the minimum when working on Radium. Especially a mask, I'd say, is essential to avoid inhaling any particles. I understand that's the main risk for Radium and its alpha radiation, which is harmless in a closed watch case with its crystal, but dangerous inside the body. I keep my watches, including one with a lot (!) of Radium on the dial and hands in a normal watch box -- I guess I should rather keep it in a well-ventilated area due to its Radon gas emissions. My approach is based on some researching on the topic + an innate fear of radioactivity (maybe based on my mother's stories of Chernobyl fallout/rain when I was a small child). -

Should I be worried about radium?
mikepilk replied to danizzz's topic in Chat About Watches & The Industry Here
-
Should I be worried about radium?
stylo9 replied to danizzz's topic in Chat About Watches & The Industry Here
Radium hands. treat with respect Radium dial numbers, usually under the varnish coat and also not nearly as active when tested. Basically don't eat it and wash hands after dealing with it Ladies who died from radiation poisoning from watch industry were ingesting large amounts daily for years -
Radium is very dangerous if it gets into your body. This can happen by ingestion, inhalation or contact with broken skin. Radium lume dust is more mobile than flakes, and therefore more likely to find its way into the body unless the right precautions are taken. The greater the dose of radium absorbed into the body, the more danger it poses, whether it’s in the form of dust or flakes makes no difference once it’s inside you. The radium present on a watch dial won’t kill you outright. Once absorbed, it is chemically very similar to calcium, and the body treats it like calcium and stores it in your bones. From there within your body, it’s alpha particle radiation plays havoc with nearby cells’ DNA, causing mutations as cells divide. The end result is often cancer. Some watch brands used radium into the 1970s, so if you are working on watches with lume that were made before 1980, you should assume that the lume contains radium unless you’ve proven otherwise with a Geiger counter. Geiger counters are now cheap enough that anyone working with vintage watches would be wise to buy one and learn how to use it. The absorption of a tiny quantity of radium can potentially have life changing impacts upon your health. Best Regards, Mark
-
Hello y'all, I have a quick question regarding this little beater. Does this seem radioactive to you? As you can see, the lettering on the logo and some description of the watches movement were brushed off by one of my water dipped q-tips. I had no idea it was that sensitive, and it also makes me think that this dial is super old. Is it possible that it's radioactive? If its only a little, then I'm ok with that. But I'm not sure. I think it's fairly possible that this watch predates the 1960's when radium was not known to be bad. Feel free to bash me for the dial cleaning job or let me know if it's safe or not. Thank you, -Matt
-
Not at all, they presumably are using tritium - I've just never seen a watch with it, in person. All mine are are either no lume, radium or phosphor (only glowing after exposure to light). [Other than the digital ones!] I have other things with tritium "glow", which have noticeably faded quite a bit in the twenty plus years I've had them, from very obvious to rather dim. I'd guess tritium is mainly used on more recent, high end watches? I buy either vintage ones that look interesting, or ebay juck packs that tend to have either very well worn vintage or cheap digital and "bling" grade stuff..
-
Excellent post and advice @Mercurial I agree with everything you say. Yes, this is important to understand. The good thing is that you can start pretty slowly and then if your interest grows, increase the number of tools. I wouldn't be surprised if I've spent the equivalent of one or two good Rolex Submariner watches since I started eight years ago. The nice thing is that I appreciate my tools at least as much as my watches. There is something truly magical about using a well-used patinated Swiss quality tool that a skilled watchmaker used for decades. Again, excellent advice, and when you want to go wristwatch size but still not take any financial risks I'd recommend Vostok 24xx movements such as Vostok 2409. Most modern watches, less than 60/70 years or so, seem to secure the impulse jewel with friction only so that should be safe to rinse in IPA (isopropanol, not Indian Pale Ale). I've never handled Radium but Kalle Slaap made an interesting video about it some time ago. As I understand it, it's the dust that can "kill" you. https://youtu.be/Z-A-e8zSoOM
-
As long as you don't grind the stuff up and blow it around, you should be fine. Use a dust mask if in doubt. The things I'm very careful of are: Radium lume - even the stuff that is visually completely dead and inert is still highly radioactive; it's the fluorescent part that decays, not the radium. A single speck inhaled or ingested can cause cancer, so store parts in zip bags and wear a dust mask & wipe your work area down after handling anything that uses it. A proper geiger counter is a good investment if you plan on working with vintage watches, so you can check for it & take appropriate precautions. "One dip" & equivalents - the original type & the generic PERC dry cleaning fluid (Tetrachloroethylene / perchloroethylene) which is what the original one dip was mostly made of. That's toxic, a known carcinogen. Use in very good ventilation only & keep it sealed whenever possible. The newer B-Dip is presumably a safer replacement.